-
Serial Vs Parallel Dilution Method카테고리 없음 2020. 2. 18. 20:20
Parallelism is a vitally important factor in clinical trial biomarker studies, but is still regularly overlooked in the industry. There are two definitions that I feel summarize parallelism best: describes it as “a condition in which dilution of test samples does not result in biased measurements of the analyte concentration”; whereas define it as “a demonstration that the sample dilution response curve is parallel to the standard concentration response curve”. Essentially it means that when a test sample is serially diluted to result in a set of samples having analog concentration that falls within the quantitative range of the assay, there is no apparent trend toward the increasing or decreasing estimates of analyte concentration over the range of the dilutions. Take note, this is different from dilutional linearity. You can think of it this way:.

Serial Vs Parallel Port
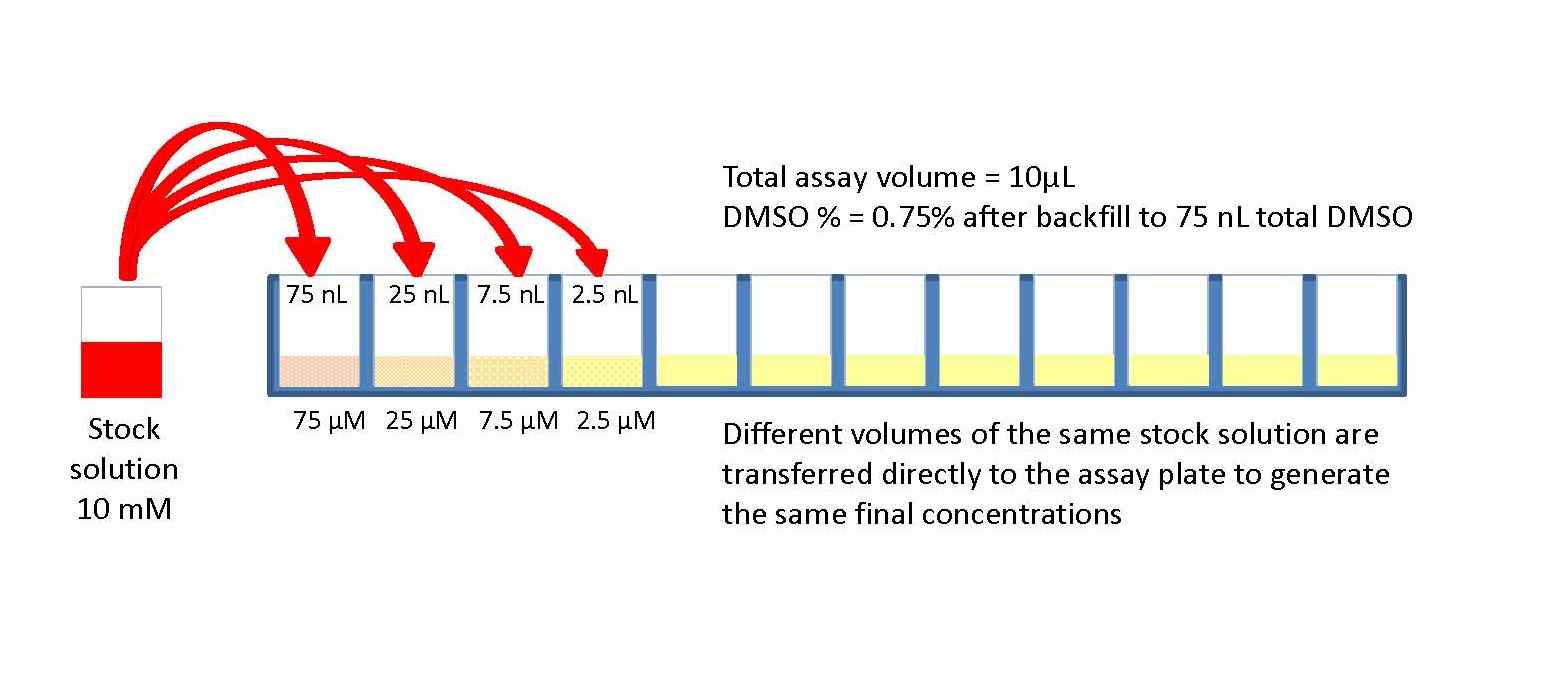
When assessing dilutional linearity, the question is – If I spike the matrix with my drug (analyte) and then dilute the sample, will it recover within an acceptable level?. When assessing parallelism, the question is – If I take a matrix with a high endogenous level of the analyte of interest and then dilute the sample, will it recover within an acceptable level?You also may be asking these questions:How do I evaluate parallelism?There is not official guidance on how to evaluate parallelism but there are a few general industry practices. The general industry practice is that you should screen at least 6 samples with a high level of analyte, but that can vary from company to company so the industry-wide range is usually between three and ten. Additionally, when performing serial dilutions (usually 2-fold) the objective should be to obtain at the least three dilutions falling within the assay range—although this standard is somewhat assay and platform dependent.
For example, on an ELISA getting three to four diluted points is typical but on an MSD or DELFIA you may be able to get six or more.When in the assay development process should parallelism be investigated?Parallelism assessment is one of the key parameters for evaluating biomarker studies and should be initiated early during the assay development stage. There is no need to wait for incurred samples to be available. It is possible to screen samples with high endogenous levels to yield some preliminary information regarding the MRD, assay selectivity, and potential LLOQ. By the pre-study validation stage, parallelism and assay limitations should be determined so that final evaluation and documentation of any issues can be completed. Now, in-study validation is not required for exploratory biomarkers unless the disease state matrix was never tested or not available up to that stage.
Serial Dilution Method In Microbiology
However, late stage biomarker studies done with an intention to develop diagnostics and/or end-point biomarkers may require assessment of parallelism at this stage.What are the acceptance criteria for biomarker parallelism assessment?Once again there are no clear requirements, but there is a general industry standard. The most commonly used is that the CV must be less than or equal to 30% amongst the in-range measurements back calculated concentration to neat concentration, although some labs are more stringent and set this measurement at 25%.

A more qualitative criterion is that there is no trend observed with increasing sample dilution. Overall, published acceptance criteria for parallelism assessment is still in its infancy and what you do with this important information depends on the intended use of the assay. Stringency may be set tighter or looser as long as the scientific rationale is justified and documented.How do I learn more?In a webinar I recently hosted for Bioanalysis Zone, I cover in more detail the considerations for evaluation of parallelism in biomarker assays, and also present several case studies showing how to interpret parallelism data. You can download the recording, entitled “Considerations for Evaluation of Accuracy, Parallelism, and Reagent Lot-to-Lot Variability in Single and Multiplex Biomarker Ligand-Binding Assays”,.Also feel free to with any questions you may have regarding parallelism assessment for your biomarker studies.